http://www.chemistrymag.org/cji/2008/10c058pe.htm |
Dec.
1, 2008 Vol.10 No.12 P.58 Copyright |
Li
Zhengping, Gao Ruiguang, Lv Xuechong, Liu Xiuxian
(College of Chemistry and Environment Science, Hebei University, Baoding
071002)
Abstract A new assay of horseradish
peroxidase (HRP) is proposed by using of resonance light scattering (RLS). The oxidation
reaction of phenol with hydrogen peroxide (H2O2) catalyzed by HRP
results in a great enhancement of RLS intensity in the wavelength range from 200 nm to 700
nm characterized by the RLS peak around 313 nm. The enhanced intensity of RLS at 313 nm is
proportional to the concentration of HRP in the range of 0.05~5.0×10-7 g/mL
and the detection limit (3s,
n=11) is 1.1×10-10 g/mL. Therefore, a simple and sensitive method for HRP
determination is established.
Keywords Horseradish peroxidase (HRP); Phenol; Resonance light scattering (RLS)
1 INTRODUCTION
The enzymatic assays have been widely used in analytic biochemistry because of their
rapidity and high selectivity[1,2]. Horseradish peroxidase (HRP) catalyzed
reaction is one of the most widely used enzymatic reactions in bioanalytical chemistry,
such as enzyme-linked immunoassy. The characteristic of HRP was systematically studied
using hydrogen peroxide (H2O2) as an oxidizing agent and various
substances as substrates. A variety of techniques, such as spectrophotometry,
chemiluminescence, fluorescence and electrochemistry, have been used for HRP detection[3-5].
However, to the best of our knowledge, resonance light scattering (RLS) technique has not
been proposed for HRP detection.
Recently, RLS has become a new interesting technique for determination
of micro amounts of biomolecules[6], such as nucleic acids[7],
proteins[8], and drug[9]. The RLS methods are very attractive
because high sensitivity can be obtained with a common spectrofluorimeter by using
inexpensive reagents.
In this paper, it is found that the oxidation reaction of phenol with H2O2
catalyzed by HRP results in a great enhancement of RLS signals. The enhanced RLS
intensities at 313 nm are proportional to the concentration of HRP in the range of
0.05~5.0×10-7 g/mL and the limit of detection (LOD) of 1.1×10-10
g/mL can be achieved without preconcentration.
2 EXPERIMENTAL
2.1. Apparatus
The RLS spectra and intensity were measured with a Hitachi F-4500 Fluorescence
Spectrophotometer (Tokyo, Japan) equipped with a quartz cell (1cm×1cm). A QL-901 Vortex
mixer (Jiangsu Clinical Instruments Plant, Haimen, China) was employed to blend the
solutions.
2.2 Reagents
A 1.0 mg/mL stock solution of HRP (300 units/mg, Xin Jing Ke Biotechnology Co. Ltd.
Beijing, China) was prepared by dissolving 1.0 mg HRP in 1 mL sterilized water and stored
in a refrigerator at 4ºC. Phosphate buffered saline (PBS, pH 6.4) was containing 0.3
mol/L NaCl and 10 mmol/mL sodium phosphate buffer. 0.625% (m/v) phenol was prepared by
dissolving 0.625 g phenol in 100 mL water. HRP working solution was prepared by diluting
the stock solution immediately prior to use. 0.6% (v/v) H2O2 was
prepared by dissolving 30% H2O2.
All reagents were of analytical grade without further purification.
Doubly distilled water was used throughout expect preparation of HRP using sterilized
water.
2.3 Procedures
In a 10 mL colorimetric tube, 1.5 mL of phenol (0.625%, m/v), 1.5 mL of PBS buffer (pH
6.4), a certain volume of HRP working solution, 50 mL of H2O2 (0.6%, v/v) were added in turn. The
mixture was diluted to the mark with water, and stirred thoroughly. After incubation at
room temperature for 30 min, 200 mL citric acid (0.3 mol/mL) was added in to stop the reaction. Then
the RLS spectra were measured against the blank solution treated in the same way without
HRP.
The RLS spectrum was obtained by scanning simultaneously with the same
excitation and emission mono- chromators of F-4500 spectrofluoro- meter from 200 to 700
nm. The intensity of RLS was measured at 313 nm. The silt width and PMT voltage of the
measurements were 5 nm and 400 v, respectively.
3 RESULTS AND DISCUSSION
3.1. Spectral Features of HRP
Fig. 1 shows the RLS spectra of the mixture of phenol and H2O2 in
the absence and in the presence of HRP. In the absence of HRP, the mixture has a
weak RLS peaks at 280 nm, which should be produced by phenol molecules. Up adding HRP in
the mixture solution, HRP catalyzes the reaction of phenol and H2O2
to produce the free radicals of phenol, which cause the polymerization and produce
polyphenol products. According to the RLS theory[10,11] the increase molecular
size and the conjugated polymer structure of polyphenol products would result in greatly
enhanced RLS. From Fig. 1, one can see that the RLS peak located at 313 nm and the RLS
intensity can be greatly increased with increasing the HRP concentration, which implies
that the RLS technique can be applied to sensitively determine HRP.
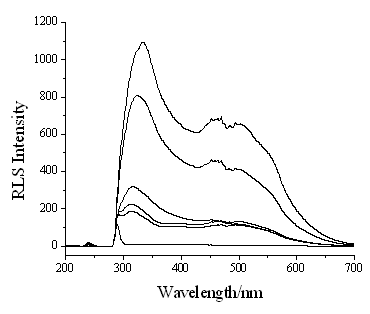
Fig.1 Resonance light scattering spectra of HRP-phenol system. HRP
(g/mL), from bottom to top 0, 5×10-9, 1×10-8, 1×10-7,
5×10-7, 1×10-6, respectively. 0.09% phenol, 0.003% H2O2.
3.2 Optimization of the general procedure
The pH value of the reaction solution plays an important role for the HRP-catalyzed
polymerization because the enzyme activity can be greatly affected by the pH value. Fig. 2
shows the influence of the pH value on the RLS intensity produced by 1×10-8
g/mL HRP. With increasing the pH value in the pH range of 5.8-8.0 adjusted by PBS buffer,
the RLS intensity firstly increases and then decrease. The maximum RLS intensity can be
obtained at pH 6.4. Therefore, pH 6.4 was chosen for use to determine HRP.
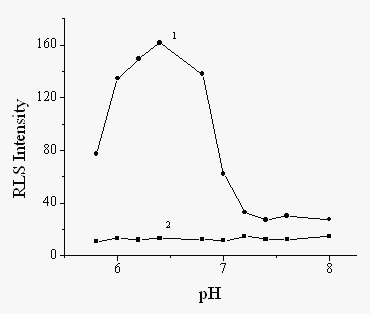
Fig.2 Influence of the pH value on RLS intensity. HRP (g/mL): 1, 1×10-8, 2, 0. 0.2% phenol, 0.006% H2O2.
The effect of ionic strength was also examined by the addition of NaCl in the PBS buffer solution. A little effect of RLS intensity with the NaCl concentration in the range of 0.015-0.075 mol/L when1.5 mL PBS buffer was used in the 10 mL reaction solution. So the PBS buffer solution containing 0.045 mol/L NaCl was used in the subsequent work.
The concentration of phenol has great effect on the formation of the polyphenol. The influence of phenol concentration on the RLS intensity was investigated in the range of 0.125%-1.25%. As can be seen from Fig. 3 that the RLS intensity are stable when the concentration of phenol was in the range of 0.02%-0.2% and the enhanced RLS intensity reaches its maximum at 0.09%. So 0.09% phenol was selected for subsequent work.
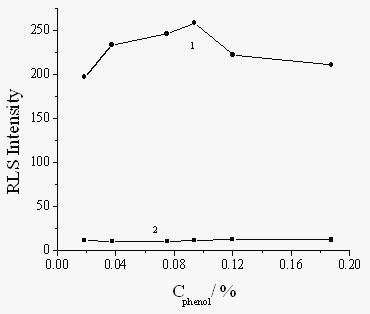
Fig.3 Effect of concentration of phenol on RLS intensity. HRP (g/mL): 1, 1×10-8, 2, 0. 0.006% H2O2.
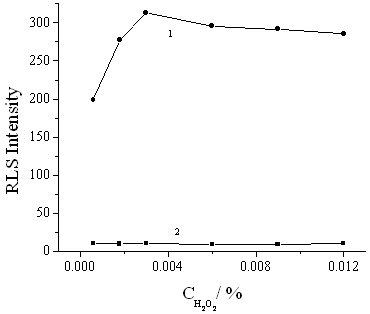
Fig.4 Effect of concentration of H2O2 on RLS intensity. HRP (g/mL): 1, 1×10-8, 2, 0. 0.09% phenol.
The effect of H2O2 concentration on the RLS intensity was also studied. As shown in Fig.4, with increasing the H2O2 concentration, the enhanced RLS intensity was increased when H2O2 concentration was less than 0.003%, and decreased when the concentration was greater than 0.003%. So 0.003% H2O2 was selected as the optimum in this assay.
The influence of the incubation time of the HRP-phenol solution on the intensity was also investigated. The RLS intensity reached its maximum after the solutions were mixed 30 min, and remained a constant at least for 30 min. The results indicated that the stability of the RLS signal is practical for the determination of HRP.
3.3 Calibration and detection limits
According to above standard procedure, the calibration curve for HRP determination is constructed under the optimal conditions. There are good linear relationships between the enhanced RLS intensity (IRLS) and the HRP concentrations in the range of 0.05~5.0×10-7 g/mL (Fig. 5). The correlation equation was IRLS=184.6 + 1.14 CHRP, the correlation coefficient r=0.9996, and the detection limit (3s, n=11) is 1.1×10-10 g/mL.
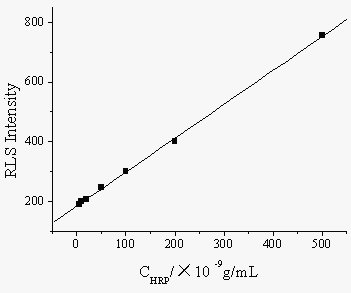
Fig.5 Relationship between enhanced RLS intensity and concentration of HRP.
4 CONCLUSIONS
In this contribution, we demonstrate that HRP catalyze the oxidation reaction of
phenol with H2O2 can form a stable associated complex polyphenol,
which produces the strong RLS signal. The enhanced RLS intensities are proportional to the
concentration of HRP in a wide range. Based on this, a highly sensitive and very
convenient RLS method for HRP determination can be developed. Therefore, the proposed RLS
method may open up a new possibility for HRP determination and has great potential
applications for bioanalytical chemistry.
REFERENCES
[1] G. G. Guilbault, P. J. Brignac, M. Zimmer, Anal. Chem., 1968, 40: 190.
[2] G. G. Guilbault, P. J. Brignac, M. Juneau, Anal. Chem., 1968, 40: 1256.
[3] B. Tang, Y. Wang, H. Liang, et al., Spectrochim. Acta. A, 2006, 63: 609.
[4] Y. Liang, H. H. Lin, J. W. Yuan, Acta. Univ. Med. Tongji (Tongji Yike Daxue Xuebao),
1998, 27: 445.
[5] M. L. Feng, Z. J. Zhang, X. R. Zhang, Chin. J. Anal. Chem. (Fenxi Huaxue), 1994, 22:
788.
[6] C. Z. Huang, Y. F. Li, Anal. Chim. Acta., 2003, 500: 105.
[7] C. Z. Huang, K. A. Li, S. Y. Tong, Anal. Chem., 1997, 69: 514.
[8] Z. P. Li, C. H. Liu, Y. Q. Su et al. Chem. J. on internet, 2005, 12: 83.
[9] P. Feng, W. Q. Shu, C. Z. Huang et al. Anal. Chem., 2001, 73: 4307.
[10] R. F. Pasternack, C. Bustamante, P. J. Collings, et al. J. Am. Chem. Soc.,
1993, 115: 5393.
[11] R. F. Pasternack, P. J. Collings, Science, 1995, 269: 935.
[12] C. Z. Huang, K. A. Li, S. Y. Tong, Anal. Chem., 1996, 68: 2259.
[13] Y. Q. Cheng, Z. P. Li, Y. Q. Su et al. Talanta, 2007, 71: 1757.
[14] N. R. Curvetto, D. Figlas, A. Brandolin et al. Biochem. Engin. J., 2006, 29:
191.
[15] J. A. Akkara, M. S. Ayyagari, F. F. Bruno, Tibtech., 1999, 17: 67.
[16] L. P Wu, Y. F. Li, C. Z. Huang et al. Anal. Chem., 2006, 78: 5570.
基于苯酚与
H2O2的氧化反应共振光散射技术测定辣根过氧化物酶李正平,高瑞广,吕学冲,刘秀贤
(保定 河北大学化学与环境科学学院,071002)
摘要 本文建立了一种高灵敏度的测定辣根过氧化物酶(HRP)的共振光散射分析方法。HRP催化苯酚和H2O2反应生成酚类共轭聚合物,并在313nm处产生强的共振光散射峰。HRP浓度在0.05~5.0×10-7 g/mL范围内与光散射强度呈良好的比例关系,对HRP的检出限(3s,n=11)为1.1×10-10 g/mL。
关键词 辣根过氧化物酶,苯酚,共振光散射