http://www.chemistrymag.org/cji/2009/111004pe.htm |
Jan.
9, 2009 Vol.11 No.1 P.4 Copyright |
Synthesis and characterization of Fire retarded poly (DAMT -co-acrylonitrile)
Jiang Aibing1, Cheng Bowen2,
Ren Yuanlin1,2, Li Zhenhuan2,
Lu Youcai2
(1School of Textile of Tianjin Polytechnic University, Tianjin 300160; 2
Tianjin Municipal Key Laboratory of Fiber Modification and Functional Fiber, Tianjin
Polytechnic University, Tianjin 300160, China)
Abstract In this paper
diethoxy-acrylamide methoxyl thiophosphate (DAMT) was synthesized with the reaction
N-hydroxymethyl acrylamide and O, O-diethyl phosphorochloridothioate catalyzed by cuprous
chloride in presence of triethylamine, and the flame retarded poly (DAMT-co-acrylonitrile)
was obtained though free-radically copolymerized process. The properties of copolymer were
estimated by Limiting Oxygen Index (LOI) values and thermogravimetric analysis (TGA). The
results showed that the LOI value of copolymer with 20 wt% percentage of DAMT coule reach
27vol%.
Keywords: Diethoxy-acrylamide methoxyl thiophosphate,
Polyacrylonitrile, Flame retardants, Thermal analysis, Limiting Oxygen Index
Polyacrylonitrile (PAN) is widely used in many aspects because it is easily processed into products of various shape with well flexibility and retention at break to meet the requirements of versatile and advanced applications. However, the combustion of PAN is very easy when it is ignited, which makes it difficult to be used in some ways. Thus, the flame retardant PAN is required eagerly. In order to increase the flame retardant property of PAN, flame retardants (FRs) are often needed. Usually, flame retardant PAN can be achieved through the use of comonomers which often contained halogens and is commonly referred to as a "modacrylic". These comonomers included vinylidene chloride, vinyl chloride and the corresponding bromides[1-3]. However, these "modacrylic" can form toxic halide gases and so call dioxin problem during burning. In view of the importance of environment protection, a growing demand to avoid the generation of such toxic gases during thermal degradation has led to the development of non-halogen containing flame retardant polymers [4].
In recent studies, phosphorus, nitrogen, sulfur, and silicon-containing compounds were considered as environmental friendly FRs, because they produce less toxic, smoking and corrosive gases [5-9]. Though the above described elements could be used alone as flame retardants, the FRs contains two or three fire retarded elements could provide polymers with better flame retardancy [10-12]. Up to now, few studies on the FRs which contains three or four flame retardant elements. Herein, we describe diethoxy-acrylamide methoxyl thiophosphate (DAMT) synthesized through a simple method, and the flame retarded poly (DAMT-co-acrylonitrile) was obtained free-radically copolymerized with DAMT and acrylonitrile. The results show that the incorporation of a low percentage of DAMT into copolymer has a significant effect on retarding combustion of the copolymer. 2 EXPERIMENTAL
2.1 Materials
O, O-diethyl phosphorochloridothioate was purchased from Nanjing Fine-Chemical Company Limited and was used as received. acrylonitrile (distillation before use), N-hydroxymethyl acrylamide, chloroform, triethylamine (TEA), cuprous chloride (CuCl), N,N-Dimethylformamide (DMF) and azobisiso-butyronitrile (AIBN, recrystallization from methanol) were all purchased from Tianjin chemical and reagent corporation. Polyacrylonitrile (PAN) was friendly obtained from Sinopec Qilu Petrochemical Company Limited and used without further treatment.
2.2 Characterization
1HNMR spectra were carried out on BRUKER AC-P200 spectrometer (300 MHz) with CDCl3 as a solvent, and TMS used as an internal standard. Fourier transform infrared (FTIR) spectra were recorded on a BRUKER VECTOR 22 spectrometer with KBr pellets. Differential scanning calorimeter (PERKIN-ELMER DSC7) measurements were used, and samples of approximately 5-10 mg in weight were sealed in hermetic aluminum pans and scanned in calorimeter with heating rate of 10 K/min in the range of 20-400 oC under N2 atmosphere. Thermogravimetric analysis (TGA) was performed on a Perkin-Elmer (Beaconsfield, UK) 7 Series instrument under a nitrogen atmosphere at a constant heating rate of 20 oC/min. Limiting oxygen index (LOI) measurements were performed on a Stanton Redcroft (East Grinstead, UK) FTA flammability unit in conformance with ASTM D2863 on rods of copolymers measuring 100 mm × 5 mm× 2 mm made by pressing the powdered polymer in a mild-steel die of the same dimensions. Pressing was performed by using a hydraulic press to a constant force of 3.8 × 108 Pa (55 × 103 psi, 10ton on 5cm2) for 3 min at room temperature.
Scanning electron microscopy (SEM, Quanta 200) was used to investigate the morphology of the char layer of samples being tested after the LOI test. The sample was coated with gold to make the surface conductible.
2.3 Synthesis of diethoxy-acrylamide methoxy thiophosphate
Chloroform (50 mL), N-hydroxymethyl acrylamide (10.1 g, 0.1 mol), triethylamine (14.5 mL) and cuprous chloride (0.1 g) were added into a 250 mL round-bottom four necked flask equipped with magnetic stirrer, condenser, drop funnel and thermometer. O, O-diethyl phosphorochloridothioate (16.5 mL, 0.1 mol) was dropped into flask with speed of 1 drop/min. After reaction temperature was kept at 0 oC for 3 h, reaction system was elevated to 25 oC for another 12 h. The obtained crude product was purified with chloroform, 2 wt% cold sodium hydroxide solution and distilled water, and diethoxy-acrylamide methoxy thiophosphate yield amounts to 80% (wt). The reaction route was shown in Scheme 1.
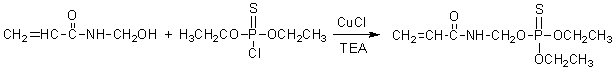
Scheme 1 The synthesis of diethoxy-acrylamide methoxy thiophosphate
2.4 Synthesis of poly
(DAMT-co-acrylonitrile)
Acrylonitrile, DAMT were added into a 250 mL four-necked flask equipped with mechanical
stirrer, thermometer and rubber pipe to introduce nitrogen gas to remove the air in flask.
AIBN (0.4 g) was dissolved in N, N-dimethylformamide, and was added into flask.
Temperature was kept at 55 oC for 6 h to obtain a milkiness liquid which was
washed with acetone to remove the unreacted monomer and by-products. The reaction route is
shown in Scheme 2.
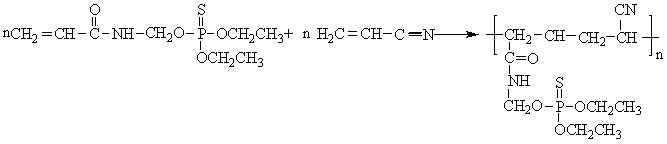
Scheme 2 The Synthesis of the poly
(DAMT-co-acrylonitrile)
3.1 Characterization of DAMT and poly (DAMT-co-acrylonitrile)
1H NMR spectra of the DAMT was shown in Figure 1. The chemistry shift at 1.2 and 1.9 ppm indicated the presence of -CH3 and -CH2-, and 4.1, 6.1, 5.6 and 4.2 might be ascribed to the existence of -O-CH2, -NH-, -CH= and (=CH2) groups.
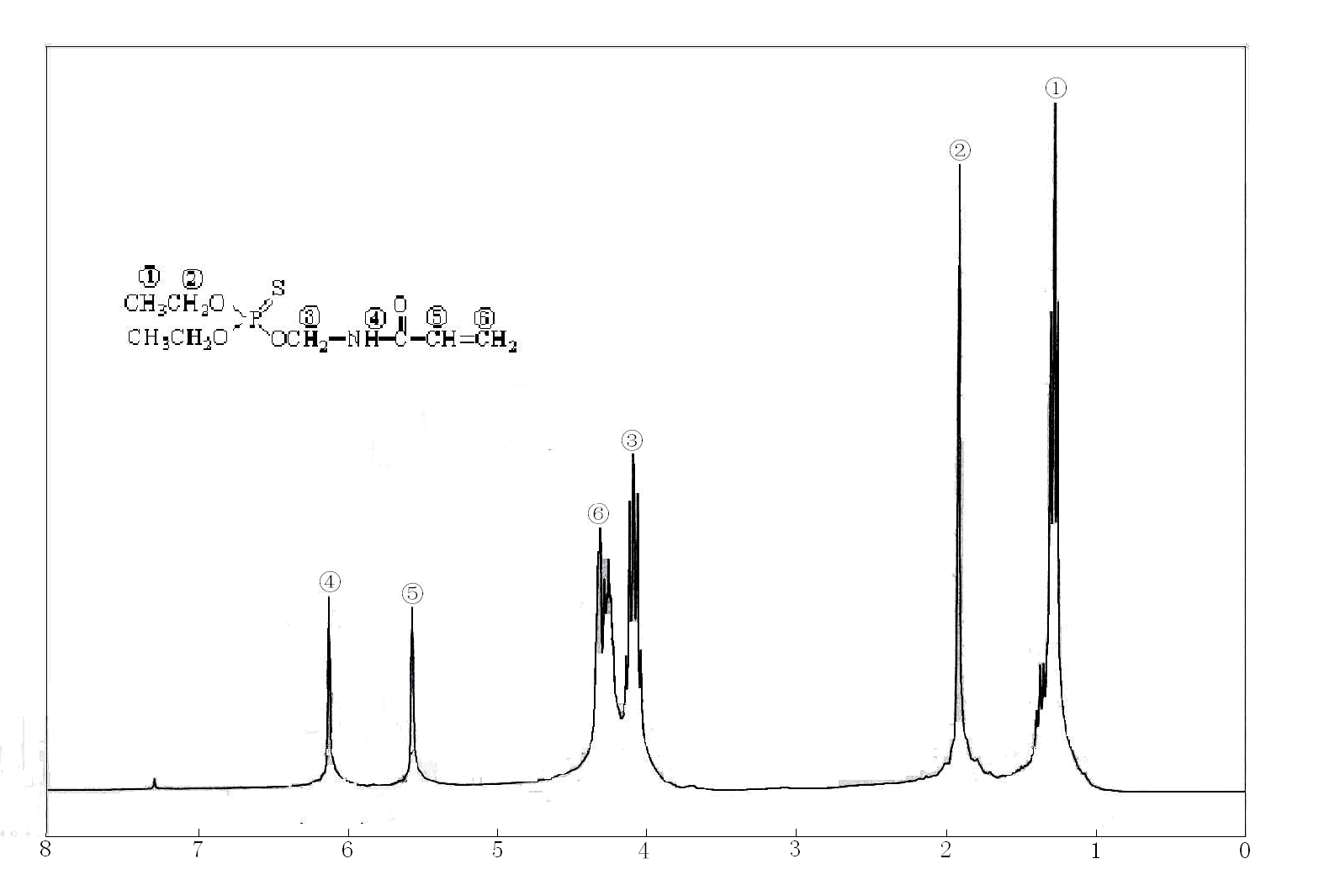
Figure 1 1H NMR spectrum of the title compound
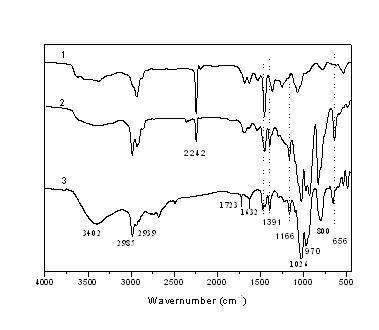
Figure 2 FT-IR spectrum of PAN (1) and poly (DAMT-co-acrylonitrile) (2) and DAMT (3)
FTIR spectra of DAMT, poly (DAMT-co-acrylonitrile) and PAN were shown in Figure 2. The bands at 656 cm-1 can be ascribed to the bending vibration of P=S, and the bands at 1026 cm-1 and 970 cm-1 were attributed to the P-O-C presence[13]. 3402 cm-1, 2985 cm-1, 1464 cm-1, 1391 cm-1, 800 cm-1 were observed, which were ascribed to the existence of N-H and CH2CH3 respectively. The appearance of band at 1723 cm-1 and 1632 cm-1 indicated that C=O and C=C existed in the DAMT. The FT-IR spectrum of the characteristic stretching vibration peak of C≡N group (2241 cm-1) appeared in both two curves of poly (DAMT-co-acrylonitrile) and PAN, however, in the poly (DAMT-co-acrylonitrile) spectrum, the bands at 1028 cm-1, 643 cm-1, and 3433 cm-1 were observed, which might be ascribed to the stretching vibration of CH2CH3, bending vibration of P=S, and N-H, respectively. The characterized results implied that diethoxy-acrylamide methoxy thiophosphate have been copolymerized with acrylonitrile.
Figure 3 shows the DSC curves of PAN and the fire retarded copolymer(FRPAN). The PAN decomposes from 270 oC to 340 oC, while the fire retarded copolymer begins to decompose at 215 oC, and terminated at 320 oC. It can be found that the exothermic peak of PAN is narrower than that of copolymer. This indicated that the flame retardant copolymer changed the cyclization mechanism of PAN. That is the cyclization reacion mechanism changed from free radical mechanisim for PAN to ionic mechanism for FRPAN[14-15]. The results show that the DAMT in the copolymer decomposes previously, which results in char forming to prevent more heat release, thus the decomposition temperature arises accordingly.
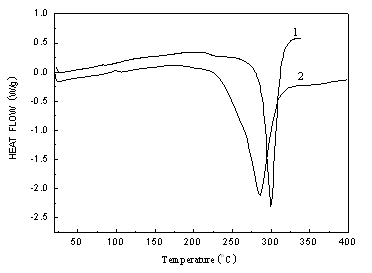
Figure 3 DSC curves of PAN (1) and poly (DMAT-co-acrylonitrile) (2)
The TG-DTG curves are
listed in Figure 4. The TG curve of PAN has three weight loss stages, while the TG curve
of the fire retarded copolymer has four stages of weight loss. For
the TG-DTG curves of PAN, the first decomposition step takes place from room temperature
to about 140 oC, which is the dehydration process[16], with about 3
wt% weight loss, and the fasted weight loss temperature lies in 74 oC. During
the second and the third weight loss steps, the weight loss rate is very fast, which takes
place from 290 to 477 oC having about 50 wt% weight loss. The second weight
loss stage locates between 290 oC and 360 oC, and the temperature of
the fastest weight loss rate is 312 oC. While the third weight loss stage is
situated at 360 oC to 477 oC, and 417 oC is the fasted
weight loss temperature. Above 200 oC, the nitrile group begains to cyclize and
exothermic reaction occurs[17], In this period,
HCN, NH3, H2 can be formed[18], and the degradation is
very acutely. Above 500 oC, the weight loss is very slow, which may be due to
the char-oxidation of the PAN residue[19] As for
the fire retarded copolymer, there are four main weight loss stages. In the first stage,
weight loss occurs between 42 oC and 87 oC, which is owning to the
dehydration with 0.2 wt% weight loss. The second stage occurs from 122 oC to
247 oC with 7.4 wt% weight loss, which may be because of the decomposition of
the fire retarded co-monomer, i.e. diethoxy-acrylamide methoxyl thiophosphate. During the
third period (250 oC to 370 oC), in the same way, the nitrile group
begains to cyclize and exothermic reaction occurs[17], In
this period, HCN, NH3, H2 can be formed[18], There is 22 wt% weight loss at the fourth stage (372 oC
to 500 oC ). In this period, the cyclized structure of the copolymer begains to
dehydrogenate, then to a fully aromatized char, and H2 can be released[16,18,20].
From the fire retarded copolymer, it can be seen that the weight loss rate during the
third and the fourth is very slower than that of PAN, for the existence of the fire
retarded copolymer diethoxy-acrylamide methoxyl thiophosphate, which decompose to
phosphoric acid or pyrophosphoric acid at high temperature to reduce the degradation of
PAN substrate. Above 800 oC, the weight loss rate is as slower as that of PAN,
and char residue is about 44.6 wt% at 1000 oC, which indicates that DAMT has
excellent fire retardance for PAN.
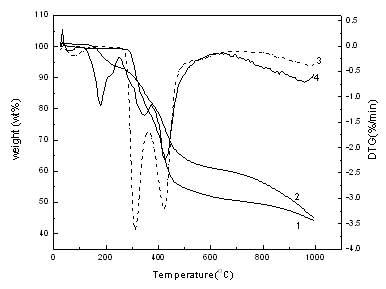
Figure 4 TG curves of PAN (1) and poly
(DMAT-co-acrylonitrile) (2)
DTG curves of PAN (3) and poly (DMAT-co-acrylonitrile) (4)
3.2 Flame-retardant property
The fire retarded copolymer was dissolved in the solution of sodium thiocyanate at the
concentration of 50 wt% to get the fiber-spinning solution, and the solution was
transfered onto a glass plate to get a thin film. After the film was dipped into water for
20 min, it was taken out of water and dried at 50 oC in air for 12 h. The LOI
results showed that the LOI value of the fire retarded copolymer was 26 vol% with 25 wt%
fire retarded comonomer, and with the comonomer percentage increasing, the LOI value
increases according, while the LOI value of untreated PAN was 17.5 vol%.
3.3 Morphology of the char residue of the fire retard PAN
The SEM micrographs in Fig. 5 show the morphological structures of the char residue of
poly (DAMT-co-acrylonitrile). The morphology of the surface view of the char layer was
shown in Fig. 5a. It could be seen that the outer surface was compact and had many
bubbles. While the morphology of cross-section view of the char showing in Fig. 5b. was
honeycomb structrue, it may be because of the gases produced in the process of
degradation, which bumped the substrate to form the phenomenon, and could provide a
barrier to prevent the transfer of heat and mass during a fire.
4 CONCLUSION
Poly (DAMT-co-acrylonitrile) was successfully synthesized through
free-radically process. The flammability property of the fire retarded copolymer was
estimated by Limiting Oxygen Index (LOI) values, and the result showed that the LOI value
of the fire retarded copolymer could amount to 26 vol% with 10 wt% DAMT.


Figure 5 SEM micrographs of fire retard
polyacrylonitrile char residue ( a. surface view of the fire retarded PAN; b.Cross-section
view of the fire retard PAN)
ACKNOWLEDGMENTS: The authors are grateful for the financial support of the Natural Science Foundation of Tianjin Education Committee (20070607).
REFERENCES[1] P .Wyman, V .Crook , J .Ebdon, B .Hunt and P .Joseph, Polym. Int., 55, 764 (2006).
[2] R. C. Nametz, Ind. Eng. Chem., 62, 41 (1970).
[3] J .M. Catala and J .Brossas, Prog. Org. Coat., 22, 69 (1993).
[4] A .B .Morgan and J. M. Tour, Macromolecules, 31, 2857 (1998).
[5] C. S. Wu, Y. L. Liu and Y .S .Chiu, Polymer, 43, 4277 (2002).
[6] Y. L .Liu, Polymer, 42, 3445 (2001).
[7] G. H .Hsiue, W. J .Wang and F. C. Chang, J. Appl. Polym. Sci., 73, 1231 (1999).
[8] C. Jayakody, G. L. Nelson, U. Sorathia and S. Lewandowski, J. Fire Sci., 16, 351 (1998).
[9] W. J. Wang, L. H. Perng, G. H. Hsiue and F. C. Chang, Polymer, 41, 6113 (2000).
[10] Y. L. Ren, B. W. Cheng, J. S. Zhang, H. J. Zang, W. M. Kang and C. K. Ding, Acta. Chimica. Sinica. (in Chinese) 65, 2034 (2007).
[11] C. S. Wu, Y. L. Liu and Y. S. Chiu, Polymer 43, 4277 (2002).
[12] S. Zhang and A. R. Horrocks, Prog Polym Sci 28, 1517 (2003).
[13] M. J. Tsafack and J. Levalois-Grutzmacher, Surf. Coat. Technol, 200, 3503 (2006).
[14] Xue-ping Wu, Yong-gang Yang, Li-cheng Ling, Fu He, New Carbon Materials, 18, 196 (2003).
[15] Wang-xi Zhang, Yan-zi Wang, Jian-jun Liu, Hua-su Cai, Polym. Mater. Sci. Eng., 16, 76 (2000).
[16] Ying-hua Qiu, Tong-hua Wang and Cheng-wen Song, Carbon Tech., 24, 4 (2005).
[17] M. M. Coleman and R. J. Petcavich, J. Polym. Sci. Polym. Physics, 16, 821 (1978).
[18] Mao-zhang Wang and Fu He, Manufacture characterized and Application of Carbon Fiber, Beijing, Science Press, 50, (1998).
[19] M. E. Hall, J. Zhang and A. R. Horrocks, Fire Mater., 18, 231 (1994).
[20] J.Zhang, A.R.Horrocks and M.E.Hall, Fire Meter., 18, 307 (1994)
DAMT与丙烯腈阻燃共聚物的合成及表征
蒋艾兵1,程博闻2,任元林1,2,李振环2,鲁友财2
(1天津工业大学天津市改性及功能纤维天津市重点实验室,天津
300160;2天津工业大学纺织学院,天津
300160)
摘要 以N-羟甲基丙烯酰胺与二乙氧基硫代磷酰氯为反应物,氯化亚铜和三乙胺为催化剂,合成了阻燃剂二乙氧基丙烯酰胺甲氧基硫代磷酸酯(DAMT)。通过与丙烯腈自由基共聚得到了阻燃聚丙烯腈共聚物,将共聚物进行了极限氧指数和热分析测试,结果表明:当阻燃剂在共聚物中所占质量分数为20%时,极限氧指数(LOI)值可达27%。
关键词 二乙氧基丙烯酰胺甲氧基硫代磷酸酯,聚丙烯腈,阻燃剂,热分析,极限氧指数