| Int. J. Mol. Sci. (ISSN 1422-0067, CODEN: IJMCFK) | Special Issue: "Structure-Property/Activity Modeling of Polyphenols" The special issue belongs to the section "Physical Chemistry, Theoretical and Computational Chemistry" |  |
|---|
[Editors] [Call for Papers][Announced Papers] [Published Papers] [Leading Review Papers] [List of Keywords]
Rationale for
the Special Issue “Structure-Property/Activity Modeling of Polyphenols”
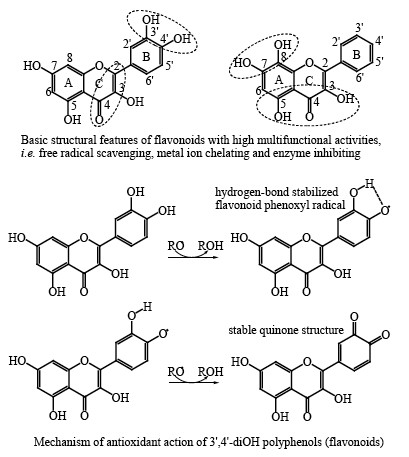 | Dear
Colleague, Polyphenols
(flavonoids, phenolic acids etc.) are abundant phytochemicals in human
diet, and evidence for their role in the prevention of various diseases
such as cardiovascular and neurodegenerative diseases and cancer is
emerging. Mechanisms involved in the health-promoting activities of
these compounds are far from clear. The goal of this special issue is to present novel results about structure-activity relationships (SAR) and quantitative structure-activity (property) relationships (QSAR/QSPR) of polyphenols, which may help in resolving the mode of actions of these food phenolics. They may also help in the design of new and efficient polyphenols, which could be used as potential therapeutic agents. With best regards, Professor Nenad Trinajstic Guest Editor |
Editors
Guest Editor
Professor Nenad Trinajstic
The Rugjer Boskovic Institute, Bijenicka 54, P.O.Box 102, HR-10002 Zagreb, Croatia
Special Issue Editorial Advisor
Dr. Dragan Amic
Faculty of Agriculture, The Josip Juraj Strossmayer University, P.O. Box 719, HR-31107 Osijek, Croatia
Special Issue Editorial Advisor
Dr. Bono Lucic
The Rugjer Boskovic Institute, Bijenicka 54, P.O.Box 102, HR-10002 Zagreb, Croatia
Keywords
Topics of special interest include, but are not strictly limited to, the following:
|
|
|
Deadline for submissions: 31 December 2008.
Call for Papers
Announced Papers
Type: Review
Title: 3D-Structure and colloidal behavior of condensed tannins as viewed by NMR and Molecular Modelling
Authors: Isabelle Pianet 1 and Michel Laguerre 2
Affiliations: 1 UMR 5255 ISM CESAMO, CNRS –Université Bordeaux 1, Talence France; 2 UMR 5248 CBMN, CNRS - Université Bordeaux 1 – ENITAB, IECB, Pessac, France.
Abstract: Download the Abstract
Manuscript ID: IJMS-25-04
Type: Full Research Paper
Title: 3D-QSAR Investigation of Synthetic Antioxidant Chromone Derivatives by Molecular Field Analysis
Authors: Weerasak Samee 1,*, Patcharawee Nunthanavanit 1 and Jiraporn Ungwitayatorn 2
Affiliations: 1 Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmacy, Srinakharinwirot University, Nakhon Nayok, 26120, Thailand; E-mail: [email protected]; 2 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok, 10400, Thailand; E-mail: [email protected]; * Author to whom correspondence should be addressed: E-mail: [email protected]

Weerasak Samee 1,*, Patcharawee Nunthanavanit 1 and Jiraporn Ungwitayatorn 2
1 Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmacy, Srinakharinwirot University, Nakhon Nayok, 26120, Thailand; E-mail: [email protected]
2 Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok, 10400, Thailand; E-mail: [email protected]
* Author to whom correspondence should be addressed: E-mail: [email protected]
Received: 17 September 2007; in revised form: 30 January 2008 / Accepted: 15 February 2008 / Published: 28 February 2008
Full Research Paper: 3D-QSAR Investigation of Synthetic Antioxidant Chromone Derivatives by Molecular Field Analysis
Int. J. Mol. Sci. 2008, 9, 235-246 (PDF format, 391K)

Zhiyong Yu 1,3, Xu Long Qin 2, Yan Yan Gu 2, Di Chen 1, Qiuzhi Cindy Cui 1, Tao Jiang 2, Sheng Biao Wan 2 and Q. Ping Dou 1,*
1 The Prevention Program, Barbara Ann Karmanos Cancer Institute, and Department of Pathology, School of Medicine, Wayne State University, Detroit, Michigan, USA
2 Key Laboratory of Marine Drug, Ministry of Education, Medical College, Ocean University of China, Qingdao, China.
3 Shandong Tumor Hospital & Institute, Breast Cancer Center, Jinan, Shandong, China
E-Mails: [email protected] (Z. Y.); [email protected] (X. L. Q.); [email protected] (Y. Y. G.); [email protected] (D. C.); [email protected] (Q. C. C.); [email protected] (T. J.); [email protected] (S. B. W.); [email protected] (Q. P. D.)
*Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel. +313-576-8301
Received: 1 April 2008; in revised form: 12 May 2008 / Accepted: 26 May 2008 / Published:
Article: Prodrugs of Fluoro-Substitued Benzoates of EGC as Tumor Cellular Proteasome Inhibitors and Apoptosis Inducers
Int. J. Mol. Sci. 2008, 9, 951-961 (PDF format, 329K); DOI: 10.3390/ijms9060961
Leading Papers and Reviews
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V; Lucic, B.; Trinajstic, N. SAR and QSAR of the Antioxidant Activity of Flavonoids. Curr. Med. Chem. 2007, 14, 827-845.
- Reis, M.; Lobato, B.; Lameira, J.; Santos, A.S.; Alves, C.N. A theoretical study of phenolic compounds with antioxidant properties. Eur. J. Med. Chem. 2007, 42, 440-446.
- Khlebnikov, A.I.; Schepetkin, I.A.; Domina, N.G.; Kirpotina, L.N.; Quinn, M.T. Improved quantitative structure–activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems. Bioorg. Med. Chem. 2007, 15, 1749-1770.
- Cabrera, M.; Simoens, M.; Falchi, G.; Lavaggi, M.L.; Piro, O.E.; Castellano, E.E.; Vidal, A.; Azqueta, A.; Monge, A.; de Cerain, A.L.; Sagrera, G.; Seoane, G.; Cerecetto H.; Gonzalez, M. Synthetic chalcones, flavanones, and flavones as antitumoral agents: Biological evaluation and structure-activity relationships. Bioorg. Med. Chem. 2007, 15, 3356-3367.
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872-2888.
- Raad, I.; Terreux, R.; Richomme, P.; Matera, E.L.; Dumontet, C.; Raynaud, J.; Guilet, D. Structure–activity relationship of natural and synthetic coumarins inhibiting the multidrug transporter P-glycoprotein. Bioorg. Med. Chem. 2006, 14, 6979-6987.
- Brand, W.; Schutte, M.E.; Williamson, G.; van Zanden, J.J.; Cnubben, N. H.P.; Groten, J.P.; van Bladeren P.J.; Rietjens I.M.C.M. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed. Pharmacother. 2006, 60, 508-519.
- Lameira, J.; Medeiros, I.G.; Reis, M.; Santos, A.S.; Alves, C.N. Structure-activity relationships study of flavone compounds with anti-HIV-1 integrase activity: A density functional theory study. Bioorg. Med Chem. 2006, 14, 7105-7112.
- Prabhakar, Y.S.; Gupta, M.K.; Roy, N.; Venkateswarlu, Y. A high dimensional QSAR study on the aldose reductase inhibitory activity of some flavones: Topological descriptors in modeling the activity. J. Chem Inf. Model. 2006, 46, 86-92.
- Sadeghipour, M.; Terreux, R.; Phipps J. Flavonoids and tyrosine nitration: structure–activity relationship correlation with enthalpy of formation. Toxic. in Vitro 2005, 19, 155-165.
- van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; van Bladeren, P.J.; Rietjens, I.M.C.M.; Cnubben, N.H.P. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol.2005, 69, 699-708.
- Zhang, H.Y. Structure-Activity Relationships and Rational Design Strategies for Radical-Scavenging Antioxidants. Curr. Comp.-Aided Drug Des. 2005, 1, 257-273.
- Zhang, S.; Yang, X.; Coburn, R.A.; Morris, M.E. Structure activity relationships and quantitative structure activity relationships for the flavonoid-mediated inhibition of breast cancer resistance protein. Biochem.Pharmacol. 2005, 70, 627-639.
- Fernández, M.; Caballero, J.; Helguera, A.M.; Castro, E.A.; González, M.P. Quantitative structure–activity relationship to predict differential inhibition of aldose reductase by flavonoid compounds. Bioorg. Med.Chem. 2005, 13, 3269-3277.
- Mukherjee, S.; Mukherjee, A.; Saha, A. QSAR modeling on binding affinity of diverse estrogenic flavonoids: electronic, topological and spatial functions in quantitative approximation. J. Mol. Struct. (Theochem) 2005, 715, 85-90.
- Rasulev, B.F.; Abdullaev, N.D.; Syrov, V.N.; Leszczynski, J.A Quantitative Structure-Activity Relationship (QSAR) Study of the Antioxidant Activity of Flavonoids. QSAR Comb. Sci. 2005, 24, 1056-1065.
- Nemeikaite-Ceniene, A.; Imbrasaite, A.; Sergediene, E.; Cenas, N. Quantitative structure-activity relationships in prooxidant cytotoxicity of polyphenols: Role of potential of phenoxyl radical/phenol redox couple. Arch. Biochem. Biophys. 2005, 441, 182-190.
- Rackova, L.; Firakova, S.; Kostalova, D.; Steferk, M.; Sturdik, E.; Majekova, M. Oxidation of liposomal membrane suppressed by flavonoids: Quantitative structure-activity relationship. Bioorg. Med Chem. 2005, 13, 6477-6484.
- Fylaktakidou K.C.; Hadjipavlou-Litina D.J.; Litinas K.E.; Nicolaides D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities Current Pharmaceutical Design. 2004, 10, 3813-3833.
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Trinajstic, N. Structure-Radical Scavenging Activity Relationships of Flavonoids. Croat. Chem. Acta 2003, 76, 55-61.
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773-781.
- Van Hoorn, D.E.C.; Nijveldt, R.J.; Van Leeuwen, P.A.M.; Hofman, Z.; M'Rabet, L.; De Bont, D.BA.; Norren, K.V. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur. J. Pharm. 2002, 451, 111-118
- Kim, D.O.; Lee, C.Y. Comprehensive Study on Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Various Polyphenolics in Scavenging a Free Radical and its Structural Relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253-273.
Webmaster [email protected]. Updated on 2 June 2008