http://www.chemistrymag.org/cji/2002/044018pe.htm |
Apr. 1,
2002 Vol.4 No.4 P.18 Copyright |
Sorption characteristics of toluene in loess soil
Chen Hui, Yang Ruiqiang, Zhu Kun#,
Jiang Mei, Zhou Wenjun
(Department of Chemistry, Northwest Normal University, Lanzhou, 730070, China; #Department
of Environmental Engineering, Lanzhou Railway University, Lanzhou, 730070)
Received Dec. 12, 2001; Supported by the National Natural Science Foundation of China (No.29977015)
Abstract The mobility of organic
compounds in soils significantly depends on their sorption–desorption behaviors. In this work, toluene as a priority pollutant
is taken to be the probe of hydrophobic organic chemicals. The sorption-desorption
equilibrium of toluene was studied on three loess soils of various physical and chemical
properties by using the batch equilibration method. Sorption isotherms could be
appropriately described by the Freundlich equation (Cs=KpCe1/n).
The desorption process, however, exhibited pronounced “hysteresis” in the soil at
every level of initially adsorbed toluene which was subjected to desorption. Some
quantitative factors for sorption were studied as well. pH values and ionic strength of
the soils had little effect on the sorption of nonionic organic compounds (NOCs). In
contrast, the soil organic matter content could make a great difference on the sorption.
Natural loess soils with a low organic matter had a limited sorptive capability for
organic chemicals. The soil retardation for migration of organic compounds was enhanced
dramatically while existing surfactants in the soil.
Keywords Mobility, Sorption, Toluene, Surfactant, Loess Soil
The manufacture, transport, utilization and
disposal of the chemicals are always resulting in the release of significant quantities of
various organic substances into soil. The role of soils as filters and chemical reactors
is beginning to dominate our conception of how to manage natural groundwater resources to
meet quality and quantity aims. The identification of soil physical and chemical processes
within the unsaturated zone leads to understanding of the mechanisms and parameters
influencing contaminant mobility within soils and aquifers. Sorption is a key process
impacting the subsurface fate and transport of the organic contaminant [1].
Downward movement of organic chemicals through the soil profiles is typically retarded by
the sorptive properties of soil constituents [2].
There are many factors influence the sorption of chemicals in soil.
Sorption of nonionic organic chemicals has been directly related to the hydrophobicity of
the materials. The physical and chemical characteristics of the sorbent also play an
important role in the sorption processes. The sorption of organic chemicals has been
directly correlated with the organic content and is thought to be the partitioning of the
compound to the soil organic layer [3]. Thus, organic chemicals tend to bind
more strongly to soils that have a higher organic content. However, the low organic matter
clays, soils and aquifer materials usually have less retardation capability for common
organic pollutants.
There has been enormous quantity of surfactants used in industrial and
household applications and they could influence the behaviors of other pollutants in soil
or water environment. Anionic and cationic surfactants usually coexist in water,
simultaneously sorb to particle suspended, and form particles with both positive and
negative charges. The sorbed surfactants affect greatly the sorption property of particles
and the transport and transformation behavior of the organic pollutants. A better
understanding of the sorption properties and mechanisms of organic compounds on the soil
mixed with surfactants can provides some essential information on surfactants affecting
the behavior of organics in soil.
Earlier studies have demonstrated that replacing the negative inorganic
exchange cation of soil minerals and subsoils with organic cations of the form [(CH3)3NR]+
result in greatly enhanced abilities of materials to decrease the transport of chemicals
through the soil [4]. These studies also suggested that aquifer materials or
subsoils could be modified in-situ by injection of cationic surfactant to create sorptive
zones that intercept and immobilize advancing contaminant plumes [5]. Coupling
contaminant immobilization with in-situ biodegradation would provide a comprehensive
restoration technology to permanently eliminate target contaminants [6].
In the present study, we have examined the three loess soils with
different physical and chemical properties. In the actual simulative conditions, a series
of modified loess soils (organosoils) were prepared by replacing the cations with both
cationic surfactant hexadecyltrimethylammonium (HDTMA) and anionic surfactant dodecyl
benzene sulfate (SDBS). Some influence factors, such as pH and ionic strength, were
discussed too. The sorptive characteristics of organosoils were compared in terms of
magnitude and mechanisms to those of natural soils.
1. EXPERIMENTAL
1.1 Samples and analysis
Loess soil samples were collected from three different uncontaminated areas in Lanzhou
City, China. Soil 1 was taken from nonagricultural area in the subsurface 60-80cm; Soil 2
and Soil 3 were sampled from the subsurface 20-30cm and 2-10cm in a cultivated area. The
samples were air-dried and passed through 2-mm sieve before being sealed into a glass
container.
The moisture contents of the “as-received”
soil samples were determined by gravimetrically over-drying
at 105℃ for 24h while soil pH values were measured
in a 1:1 (w/w) solid-deionized water mixture. The cation-exchange capacity (CEC) was
measured by Atomic Sorption Spectrometry using Ca2+ as an index cation and 0.1M
BaCl2 solution as the extracting reagent. The organic carbon content was
measured by K2Cr2O7-H2SO4 oxidation
at 180-185℃ in an oil bath according to the reported
methodology [7]. All the results tested are listed in Table1.
Table1 Physical and chemical properties of the selected loess soils
Batch
density |
Water |
pH |
CEC |
OC (%) |
Sand (%) |
Silt (%) |
Clay (%) |
|
Soil 1 |
1.38 |
5.62 |
8.61 |
3.51 |
0.201 |
18.75 |
53.85 |
27.40 |
Soil 2 |
1.52 |
6.81 |
8.23 |
5.46 |
0.945 |
25.40 |
63.10 |
11.50 |
Soil 3 |
1.47 |
7.49 |
8.14 |
6.59 |
1.452 |
22.45 |
60.10 |
17.15 |
1.2 Chemicals
All chemicals used were analytical-grade and purified further before using. The
experimental water was organic-free and twice distilled.
1.3 Analytical procedures
Analysis of toluene in the carbon disulfide extracts was made by gas chromatography with a
FID detector and packed column. Peak areas were recorded by external standards to
determine the concentration of toluene. With the detection limits 0.05mg/L, the recovery
of toluene in blanks not containing soils could reach about 95%, and was used to adjust
the sorption data. The GC conditions showed as follows: column temperature 120℃, detection temperature 200℃,
carrier gas N2 50mL/min, H2 50mL/min, Sample size 1mL.
1.4 Preparation of organosoils
A series of single-cation and anion-cation organosoils were prepared by coating loess
soils with aqueous solutions of either cationic surfactant or a mixture of anionic and
cationic surfactants.. A total of 500g previously dried soil samples were mixed with 1L
aqueous solution containing a certain amount of the surfactant meanwhile 2L distilled
water was added. The mixtures were subjected to mechanical stirring at room temperature
for 5h. Then, the modified soils were separated by centrifugation and washed repeatedly by
distilled water until bromide ions were undetectable as indicated by AgNO3.
Eventually, the soil samples were air-dried and passed through 2-mm sieve, stored in glass
bottles at room temperature for later use.
Modified soils were identified by a prefix that set the percent of the
loess soil CEC satisfied by surfactants followed by the abbreviation for the specific type
of the anionic surfactant SDBS and cationic surfactant HDTMA. For example, 100HDTMA
presented a modified-soil that had 100% of loess soil CEC satisfied by a
hexadecyltrimethylammonium cation (HDTMA+). In the cases where anionic-cationic
mixed surfactants SDBS and HDTMA were coated to loess soil, two abbreviations were used
for the categorization of the modified soils. Analogously, 100HDTMA/20SDBS identified the
anion-cation surfactant modified soil that had 100% CEC satisfied by HDTMA+ and
with extra 20% CEC that was coated by SDBS.
1.5 Procedures for sorption
Batch equilibrium isotherms studies were conducted at 25±1℃. About 10.0g soil samples were mixed with various amounts of
toluene solutions containing 0.01M CaCl2 in 50-mL amber vials capped with
Teflon-faced silicon septa. Headspace was minimized by filling the vials to the top.
Sodium azide was used as biocides at a concentration of 200mg/L. To obtain equilibrium,
the soil-water mixture was initially stirred with a vortex agitator and placed in the
shaker for continuous mixing about 24h in the dark. After equilibration, the vials were
withdrawn and centrifuged at 4000rpm for 30min. A volume of 25.00mL supernatant was taken,
then extracted by 5.00mL carbon disulfide and analyzed immediately. Meanwhile, the blank
experiments were performed to determine the loss of solute due to adsorption on glass
walls and other losses in the same condition. The solid-phase concentration was calculated
by the difference between the initial and final concentration of the tested organic
compounds in the aqueous phase.
Studies were also conducted to assess the effects of the aqueous phase
pH and ionic strength on sorption. For the pH experiments, sorption was measured as
described above, and the initial pH of the aqueous phase was adjusted to 3.0, 8.0, 12.0
respectively using either 0.1M HCl or 0.1M KOH. For the ionic strength studies, the
concentrations of the electrolyte CaCl2 in the aqueous phase were varied from
0.01M to 0.1M.
1.6 Procedures for desorption
Batch desorption isotherm experiments were performed at 25±1℃ for an equilibrium desorption contact time 24h. 10.0g contaminated
soil samples were placed in 50-mL amber vials, filled with 0.01M CaCl2 and
containing 200mg/L sodium azide solution with the minimized headspace and capped with
Teflon-faced silicon septa. The vials were vortexed for about 5min to ensure uniform
mixing and placed in the shaker for continuous shaking until the time of equilibration in
the dark, then centrifuged at 4000rpm for 30min. A volume of 10mL supernatants was
withdrawn and analyzed. The volume of the remaining slurry was made up again to 50 mL by
adding 10 mL of CaCl2 solution, equilibrated again for 24h and centrifuged. The
process of withdrawing 10 mL of the supernatant, measuring its sorption and replacing it
by 10 mL 0.01 M CaCl2 solution for reequilibration was repeated for six
successive days. The tests were repeated by described process for a desired interval, and
the data analysis was similar to the sorption processes.
2. RESULTS
2.1 Sorption isotherms
The sorption isotherms of toluene on the three loess soils are shown in Fig.1.The sorption
isotherms for the toluene were quite in agreement with Freundlich Equation:
Cs=KpCe1/n (1)
Where Cs is the equilibrium concentration of the tested chemical in the solid
phase (mg/g at dry weight), Ce is the equilibrium concentration of the tested
chemical in the solution (mg/L); Kp and 1/n are adjustable parameters used to
fit the model data. The Freundlich constants were obtained by fitting the mean of the
triplicate values measured for each of fixed concentrations of toluene to the linear form
of equation:
LogCs=LogKp+1/nLogCe (2)
Values for Kp were obtained by linear regression analysis. Kp
provides a quantitative estimate of the relative affinity of a soil for toluene. To
facilitate comparison between experiments, Kp values were normalized to the
fraction of organic carbon (fOC) of each of the soils, KOC values
were determined from the following equation:
KOC=Kp/fOC (3)
The results are listed in Table 2. Kp decreased in the order Soil 3 > Soil 2
> Soil 1, which also match with the order of decreasing organic carbon in the soils
while the sorption followed the order Soil 3>Soil 2>Soil 1. The positive of soil
organic matter on the sorption organic chemicals is well established [8].
Table 2 Measured Freundlich Kp and 1/n values for sorption of toluene.
Kp |
1/n |
R |
KOC |
OC (%) |
|
Soil 1 |
4 |
0.84 |
0.9906 |
1990 |
0.201 |
Soil 2 |
18 |
0.58 |
0.9874 |
1846 |
0.945 |
Soil 3 |
25 |
0.57 |
0.9990 |
1722 |
1.452 |
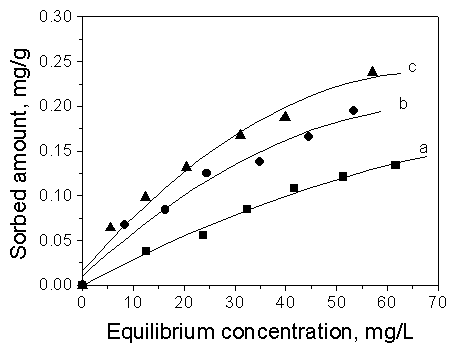
Fig.1 Comparison of sorption
of toluene on different soils.
Curve a: Soil 1; Curve b: Soil 2; Curve c: Soil 3.
2.2 Desorption isotherms
The desorption isotherms of toluene on soil 1 of three different initial concentration are
shown in Fig.2. The desorption process exhibited pronounced “hysteresis” at every level of
initially adsorbed toluene which was subjected to desorption. This was evident from the
desorption isotherms corresponding to each initial toluene concentration. The “hysteresis” was more
pronounced when desorption was carried out from higher levels of adsorbed toluene. It was
confirmed that the degree of irreversibility in the sorption-desortion process increased
with quantity of the sorbed toluene, and the similar features were also observed by
Murrary [9] for dipropetryn and prometryn.
Previous desorption studies being established are quite clear that the
sorption of organic carbon in soil is an irreversible process [10]. It is more
often the role than the exception that a large amount of the adsorbate is retained on the
soil during desorption. This leads to hysteresis when desorption is the presence of a
number of heterogeneous adsorbing sites of varying energy levels on soils [11].
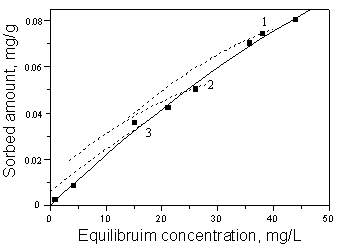
Fig.2 Desorption isotherms of the different
concentration of toluene on soil 1. (Initial concentration of toluene on soil,
Curve1:0.074mg/g; Curve2:0.051mg/g; Curve3: 0.038mg/g).
Recent research has showed that soil organic matter may comprise two principal types of heterogeneous organic domains: a "hard" carbon domain and a "soft" carbon domain [12,13]. The soft carbon domain can be envisioned as a condensed and relatively rigid organic matrix, analogous to a glassy polymer [14]. If these analogies are accurate, it is expected from the known respective sorption behaviors of the rubbery and glassy states of polymers that there are two different types of soil organic matter domains. The condensed or glassy domains, for example, would be expected to exhibit to nonlinear sorption behavior with slower rates of sorption, solute-solute competition and possible sorption-desorption hysteresis. Conversely, the highly amorphous or rubbery domain should exhibit linear sorption behavior with faster rates of sorption, no solute-solute competition and no sorption-desorption hysteresis.
3. DISCUSSION
3.1 Effects of pH and ionic strength
The effects of pH and ionic strength on sorption of toluene to Soil 1 are shown in Fig.3.
It can be seen that pH appeared to have little effect on the sorption of toluene to the
soils. Adsorbed amount increased as the ionic strength increasing from 0.01 M to 0.1M,
however, the increased extent was not significant. This may be explained that the target
organic compound was nonionic and neutral. Therefore, pH and ionic strength may not alter
its sorption mechanism.
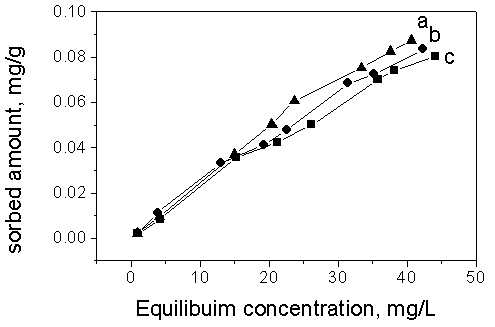 (A)
(A)
 (B)
(B)
Fig. 3 Effect of ion strength on the sorption of toluene in Soil 1
In Fig.3 (A), Curve a: 0.1M CaCl2; Curve b: 0.05M CaCl2; Curve c:
0.01M CaCl2
In Fig.3 (B), Curve 1:pH 12.0; Curve 2: pH 8.0;
Curve 3:pH 3.0
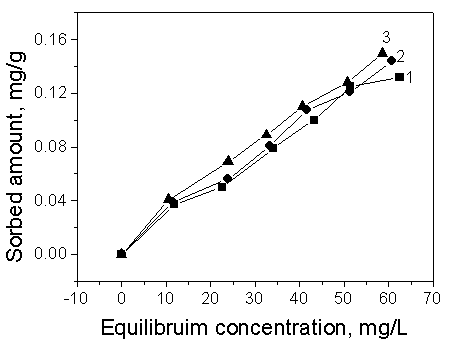
3.2 Effect of property of organic compound
For nonionic organic compounds, sorption has been directly related to the hydrophobicity
of the materials. Moreover, organic compounds with low aqueous solubility and high octanol
water partition coefficient (Kow) are more strongly adsorbed by soil than
compounds that are more soluble and have a lower Kow (Kowethylbenzene>Kowtoluene>Kowbenzene)
[15]. The results can be confirmed from Fig.4.
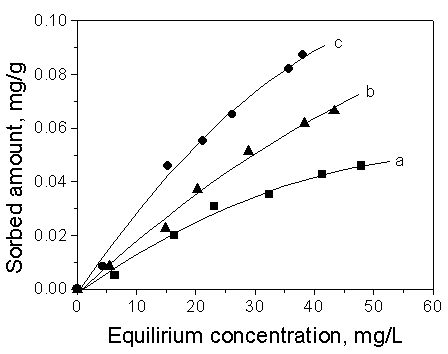
Fig. 4 Comparison of the sorption of
different organic compounds on Soil 1
Curve a: benzene; Curve b: toluene; Curve c: ethylbenzene
3.3 Effect of surfactants
Sorption isotherms for toluene at 25℃ on
single-cation and anion-cation modified soils including natural soil isotherms are shown
in Fig.5 and 6, respectively. All the experimental batch sorption data were fit to a
sorption model using nonlinear least-squares regression. From Fig.5 and 6, it could be
seen that the sorption of toluene to single cation and anion-cation organosoils both
increased gradually when the addition amount of HDTMA was varied to increase CEC from 60%
to 120% in the loess soils.
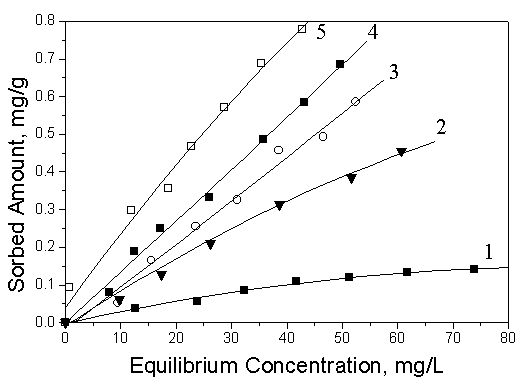
Fig.5 Sorption isotherms of toluene in
natural soil and anion-cation organosoils at 25℃
1: Natural soil; 2: 60HDTMA/20SDBS; 3: 80HDTMA/20SDBS; 4:
100HDTMA/20SDBS; 5: 120HDTMA/20SDBS
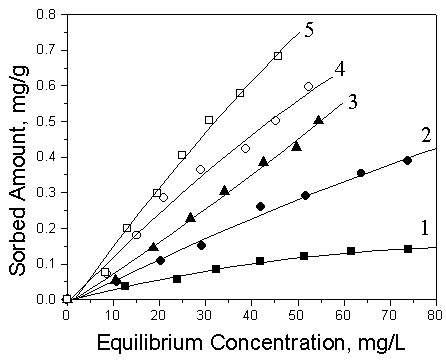
Fig. 6 Sorption isotherms of toluene in
natural soil and single-cation organosoils at 25℃
1: Natural soil; 2: 60HDTMA; 3: 80HDTMA; 4: 100HDTMA; 5:
120HDTMA
By comparison of sorption isotherms of toluene on anion-cation organosoils and on the corresponding single-cation organosoils, it was obviously confirmed that the sorption magnitudes of toluene both on 120HDTMA/20SDBS and 100HDTMA/20SDBS was larger than the corresponding single-cation modified soils with 120HDTMA, 100HDTMA. The same trend could be found in the other modified soils, which suggested that anion-cation mixed organosoils provided more powerful partition media than the corresponding single-cation organosoils. Therefore, the mixed anion-cation organosoils have the higher sorptive capacity than the corresponding single-cation organosoils for the neutral organic compounds in loess soils.
4. CONCLUSION
From this study it is summarized that both sorption and desorption of toluene by loess
soils are mainly controlled by organic carbon contents and the properties of the organics.
Usually, desorption process exhibits hysteresis effect because of heterogeneous sorption
sites in soils. The study characterized the natural loess soils, such as low organic
content, low CEC values and poor sorption capacity. The sorption of toluene depends
slightly on the pH and ionic strength of the solution. Natural loess soils with low
organic carbon contents have little sorptive capabilities for contaminants in the soil,
which conceals the potential for organic pollutants with high solubility to leach through
unsaturated zone, even have a treat on the ground water. The loess soil, while existing
surfactant, could intensely improve retardation of organic pollutants in soil matrix. The
experiments proved that the soils incorporated with surfactants had much greater sorption
effectiveness than natural soils, meantime, the organosoils with anion-cation surfactants,
had higher sorptive capacity than the corresponding single-cation organosoils. The
information obtained from the experiment is essential for the accurate modeling of organic
compounds transport and fate in the soil.
REFERENCES
[1] Totsche K U, Danzer J. J. Environ. Qual., 1997, 26 (7): 1090.
[2] Nelson S D, Farmer W J, Williams C F. J. Environ. Qual., 2000, 29 (11): 1856.
[3] Chiou C T, Peters L J, Freed V H. Science (Washington, DC), 1979, 206 (16): 831.
[4] Sheng G Y, Xu S H, Boyd S A. Environ. Sci. & Technol., 1996, 30 (5): 1553.
[5] Wager J, Chen H, Brownawell B T et al. Environ. Sci. & Technol., 1994, 28 (2):
231.
[6] Xu S H, Boyd S A. Environ. Sci. & Technol., 1995, 29 (2): 312.
[7] Li Y et al. Conventional analysis of soil chemistry. Beijing: Scientific Press, 1983,
74.
[8] Chiou C T, Peters L J, Schmedding D W. Environ. Sci. & Technol., 1983, 17 (3):
227.
[9] Leboeuf E J, Weber W J. Environ. Sci. & Technol., 1997, 31 (6): 1697.
[10] Pavlostathis S G, Mathavan G N. Environ. Sci. & Technol., 1992, 26 (3): 532.
[11] Raman S, Krishna M, Chanderasekhar P. Water Air & Soil Pollution, 1988, 40 (6):
177.
[12] Daniel R O, Raymond C L. Environ. Sci. & Technol., 1999, 33 (8): 1193.
[13] Huang W, Young T M. Environ. Sci. & Technol., 1997, 31 (6): 1703.
[14] Culver T B, Hallisey S P, Sahoo D et al. Environ. Sci. & Technol., 1997, 31 (5):
1581.
[15] Chiou C T, Shoup T D. Environ. Sci. & Technol., 1985, 19: 196.