http://www.chemistrymag.org/cji/2002/045024ne.htm |
Apr. 1, 2002 Vol.4 No.5 P.24 Copyright |
Zhang Xiaoli, Xia Zhaojie, Zhao Binxia, Jia
Zhihua
(Department of Chemical Engineering, Northwest University, Xi'an, 710069, China)
Received Dec.19, 2001; Supported by the National Natural Science Foundation of China (No. 29886001)
Abstract The effects of carbon and
nitrogen sources on secretory expression of cloned a -amylase in the culture of
recombinant yeast were studied. It shows that natural nitrogen sources are essential for
recombinant yeast to express foreign protein. A two-stage feeding strategy was developed
to achieve high plasmid stability and protein productivity by selecting suitable carbon
and nitrogen sources.
Keywords a-amylase;
recombinant yeast; fed-batch fermentation; plasmid stability
Among a variety of host systems, the yeast Saccharomyces cerevisiae is the most widely used eukaryotic host for the production of heterologous proteins. It is a well-characterized and non-pathogenic host, which has been cultivated on an industrial scale for centuries. Regulated promoters, which control gene expression by change of medium composition, addition of chemicals, or change of cultural conditions, are commonly used for foreign protein production [1]. SUC2 promoter correlates an enzyme of yeast glycogenolysis. It is repressed at high glucose concentration and enhanced at low glucose concentration [2]. Since the promoter is derepressed at low glucose concentration, it leads to lacking of carbon source in media at the production stage of foreign protein [3]. However, The carbon for microorganism biosynthesis and the required energy of cell for fulfilling physiological activity wholly are provided by carbon source. Therefore, carbon source is essential to high-level production of the foreign protein. In this research, the effects of replacing glucose with alternative carbon sources in the inducing stage and the selection of natural nitrogen sources on the productivity of foreign protein from SUC2 promoter were investigated. Meanwhile, a new feeding strategy of fed-batch was developed.
1 MATERIALS AND METHODS
1.1 Yeast and plasmid
The S. cerevisiae strain A258 (MATa , osel-1, can 1, his 4, leu 2, met 14, trp
1, ura 3) was transformed by the recombinant plasmid pNA3. Vector pNA3 [4]
was constructed by using the SUC2 promoter, Killer 28kd signal sequences, PGK
terminator, 2μm plasmid replication
origin. This plasmid contained a selectable marker gene (TRP1) and a section of the
mouse salivary a -amylase gene that was inserted between signal sequences and terminator.
1.2 Medium
Three types of medium were used in present study.
YPD medium contained 10g·L-1yeast extract, 10g·L-1peptone and
20g·L-1glucose. The second was semi-synthetic selective medium (SSM) [5]
in which auxotrophic amino acid (0.02g·L-1histidine, 0.02g·L-1leucine,
0.02g·L-1methionine and 0.02g·L-1uracil) were added supplementary.
The third medium was complete synthetic selective medium (CSM) [6].
1.3 Cultivation
The recombinant yeast strain was pre-cultured in
shaking flask containing 100 mL of medium at 30 ° C. The fed-batch cultures were carried
out in 2 L of a Bench Top Fermentor (BIOSTAT B2, B.Braun Biotch. International, Germany).
1.4 Analytical methods
The cell concentration was determined by measuring the optical density at 660 nm (OD660)
of the culture broth and converted to dry-cell L-1 by multiplying a conversion
factor, which was equal to 0.25dry-cell·OD660-1. The glucose
concentration was determined by using Glucose Reagent Kit (glucose oxidase-peroxidase method, Rong Sheng Biotech. Co. China). Plasmid stability was determined by
measuring the colony number on SSM plate as a tryptophan auxotroph. The mouse a -amylase
activity was obtained by dinitrosalcylic acid method[7]. Released of 1 mmol of glucose by
enzymatic reaction per minute was defined as 1 unit of enzyme activity. Specific activity
was defined as the enzyme activity of per mg of dry cells since natural protein, such as
yeast extract, was added into media and the amount of protein cannot be measured
accurately.


Fig.1 The effects of yeast extract on
cell growth and a-amylase
production
2.1 Precondition of expression
In this research, the SUC2 promoter regulated by glucose concentration in the culture broth was used. This promoter is repressed at high glucose concentration and enhanced at low glucose concentration. It is noted that nitrogen sources are essential for recombinant yeast to express foreign protein. Figure 1 shows the comparison between the addition of the yeast extract and without it. The former a -amylase activity was much higher than the latter. However, the addition of yeast extract was at price of losing selective pressure so as to affect the plasmid stability. How to achieve high cell density while keeping the plasmid stability becomes the key to enhancing the recombinant protein production.
2.2 Two-stage culture
Our strategy in current research was to use promoter to regulate expression system and separate cell growth and production formation into two stages. At fist stage, selective medium was used to grow cells. The promoter was repressed because of high glucose and the gene expression was restricted. When the desired high cell density was achieved, fermentation turned into the second stage. At this stage, glucose was kept at low concentration and natural nitrogen source was added. The results were listed in the Table 1. Cell density and enzyme activity with the initial glucose concentration of 7 g·L-1 and 9 g·L-1 were larger than with the concentration of 5 g·L-1, because the latter expressed the foreign protein in advance, which inhibited the growth of cells. As for low enzyme activity with the concentration of 11 g·L-1, probably because cell growth had stepped into stationary period and cells were in low activity. As the production results, expressing foreign protein in advance or postponed both resulted in lower stability of plasmid.
Tab.1 The results of different glucose concentration on two-stage cultivation
Glucose/g·L-1 |
Maximal cell mass/g·L-1 |
Maximal a -amylase activity/ U |
5 |
3.56 |
1.72 |
7 |
4.12 |
2.03 |
9 |
4.39 |
2.43 |
11 |
3.44 |
1.40 |
2.3 Selection of alternative carbon source
Two-stage fermentation may present a new problem of lack of carbon source, which is necessary energy demand for synthesizing protein. In this study, we solved the problem through the selection of alternative carbon source, which will not repress the gene expression, replacing glucose. Figure 2 shows the results of fermentation in alternative carbon sources. It is clear that feeding glycerol and lactose achieved the higher production level and cell density. As also indicates the starch was not suitable for carbon source probably because of the glucose production through the enzymic hydrolysis of a -amylase.
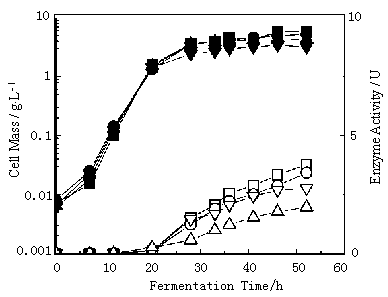
Fig.2 The effects of alternative carbon source on cell growth and a-amylase activity
Nitrogen source is essential for recombinant yeast to express foreign protein. As a comparison, four common nitrogen sources, namely yeast extract, peptone, corn steep liquor and hydrolysate of bean were studied for the level of gene expression. The results were listed in Table 2. It shows that most favorite natural nitrogen source was yeast extract. Moreover, corn steep liquor was another desirable cheap rich nitrogen source for recombinant yeast fermentation. Hydrolysate of bean was unsuited for the yeast to induce foreign protein expression because of no obvious a -amylase activity was detected.
Tab.2 The results of different nitrogen sources on two-stage cultivation
Nitrogen source |
Maximal cell mass /g·L-1 |
Maximal a -amylase activity/ U | Specific activity /U·mg-1 |
PS /% |
Yeast extract |
4.09 |
2.43 |
0.594 |
88 |
Corn steep liquor |
4.22 |
1.0 |
0.25 |
79 |
Peptone |
2.69 |
1.57 |
0.62 |
83 |
Hydrolysate of bean |
1.07 |
72 |
3 CONCLUSIONS
Fed-batch fermentation using two-stage feeding
strategy has been shown to be effective in improving plasmid stability and expression
level of cloned a -amylase. The foreign protein productivity could be further
enhanced by high cell density culture of the recombinant yeast strain.
REFERENCES
[1] Cha H J, Yoo Y J. Process Biochem., 1996, 31: 499.
[2] Cha H J, Kim M-H, Kim S H, et al. Process Biochem., 1998, 33: 257.
[3] Jang J K, Pyun Y R, Seo J M. Biotechnol. Bioeng., 1990, 36: 960.
[4] Park Y S, Shiba S, Lijima S, Kobayashi T. Biotechnol. Bioeng., 1993, 41: 854.
[5] Lin K H, Iijima S, Shimizu K, et al. Appl. Microbiol. Biotechnol., 1989, 32:
313.
[6] Rose A H. Growth and handing of yeasts, New York: Academic Press, 1975: 1.
[7] Nishizawa M, Ozawa F, Hishimura F. Agric. Biol. Chem., 1987, 52: 515.