http://www.chemistrymag.org/cji/2002/046026pe.htm |
Apr. 6, 2002 Vol.4 No.6 P.26 Copyright |
Synthesis of a porous silica-containing heteropoly acid by salt-gel reaction
Chu Lei, Zhang Yuanming, Mao Xuan#, Tang Yu
( Department of Chemistry, Jinan University, Guangzhou 510632; #Department of Biomedical Engineering, Jinan University, Guangzhou 510632, China) Received Dec. 12, 2001; Supported by the National Natural Science Foundation of China (No. 29903004) Abstract A new mesoporous structured material with Keggin anions PMo12O403- has been hydrothermally synthesized from phosphomolybdic acid, cetyltrimethylammonium bromide and tetraethyl orthosilicate as the silicon source. A solvent extract method to remove the template cations was used. The materials have been characterized by elementary analysis, X-ray diffraction, AFM, IR and nitrogen BET surface measurements. After extraction of the silica-modified salts with HCl/EtOH, the surface area of the porous material is up to 140 m2/g.
Keywords Mesoporous structured material; Keggin anion; Hydrothermal synthesis; Solvent extraction 1 INTRODUCTION
Porous inorganic materials have been studied extensively for many years due to their utility as high surface area catalysts and sorption media [1]. A significant breakthrough in the chemistry of materials was accomplished when the synthesis of MCM-41 was established by the Mobil researchers [2,3]. This new family of mesoporous materials possesses uniform and large pores (2-10nm), a large surface area over 1000m2/g and a large adsorption capacity. As a silica-based mesoporous material, M41S would be attractive as adsorbents, supports for catalysts, and hosts in inclusion chemistry, however it has several limitations due to the lattice of silica. From this viewpoint, a number of groups have successfully incorporated various metal ions such as Al, Ti, V, Ga, and B [4] into the silica framework of MCM-41. Metal oxides with porous structures have been synthesized by using structure-directing agents similar to the templates used in the synthesis of M41S. Some efforts were made to obtain porous heteropoly acids, but none was accomplished due to the low thermal stability of the heteropoly anion [5].
Recently, Stein et al. and Janauer et al. have synthesized layered mesostructured materials of (C19H42N)6(H2W12O40) and [C12H25N(CH3)3]6(H2W12O40), respectively [6,7]. These materials are of great interests because their walls are made of cluster anions, in contrast to the amorphous walls of the other materials. The tungsten, vanadium, niobium, and molybdenum oxide precursors reacting with cationic surfactants, often form similar cluster ion salts with lamellar structures [6]. In our studies we synthesized surfactant salts of phosphomolybdic acid and reacted them with tetraethyl orthosilicate (TEOS) in a “salt-gel” reaction in order to obtain a three-dimensional tunnel structure. Phosphomolybdic acid is unstable when the temperature overrun 723 K. Calcination to decompose the organic structure-directing agent will collapse the inorganic portion of the structure invariably lead to dense MoO3-x phases, hence here we removed the template by extraction with the solvent ethanol.
2 EXPERIMENTAL
2.1 Synthesis
2.1.1 Synthesis of (C19H42N)3(PMo12O40)
The solution of surfactant was prepared by dissolving CH3(CH2)15N(CH3)3Br (CTAB) in the distilled water and added the appropriate amount of H7[P(Mo2O7)6].xH2O (PMA) with vigorous stirring. After being stirred at room temperature for 1 h, the resulting mixtures were allowed to react in a Teflon-lined autoclaves at 368K from 24 to 96 h. The resultant yellow solid products were filtered, washed with distilled water, and dried in air. Elementary analysis: C, 25.08%; H, 4.86%; N, 1.22%; calculated for (C19H42N)3(PMo12O40): C,25.47%; H, 4.69%; N, 1.56%.
2.1.2 Reaction with tetraethyl orthosilicate (TEOS)
Silicate was introduced into the salts by reacting 1g of the cluster/surfactant salt with excess TEOS at 433K for 24 h in a Teflon-lined autoclave. The product was collected by filtration, washed with 95% ethanol, and dried in air.
2.1.3 Extraction of template
The cationic surfactant was removed by stirring in 150 ml of 1M HCl/EtOH at room temperature for 48h. The product was collected by filtration, washed with ethanol, and dried in air.
2.2 Product characterization
Elementary analysis for C, H, and N were carried out by Elementar Inc., vario EL, GA. X-ray diffraction (XRD) data were collected using Rigaku Corporation D/max - IIIA diffractometer with Cu Kαradiation (35kV, 25mA). Fourier transform infrared spectra were collected on a Bruker Equinox 55 FTIR spectrometer, using KBr pellets of the samples. The characterization of the fine structure of the powder was obtained on a ThermoMicroscopes Autoprobe CP Research Atomic Force Microscope. BET surface area measurements were carried out using a Quantachrome NOVA 1000 instrument. 3 RESULTS AND DISCUSSION
3.1 Infrared spectrum
The IR spectrum of (C19H42N)3(PMo12O40) is compared with those of PMA and the TEOS treated samples in Fig.1. The bands in the IR spectrum of (C19H42N)3(PMo12O40) correspond well to those of PMA, showing the typical features of Keggin anions. According to the assignments of Rocchiccioli-Deltcheff et al. [8], the four absorption bands at 1062, 957, 881, 800 cm-1 are assigned to the n(P-O), n(Mo-Ot) (Ot refers to the terminal oxygens), n(Mo-Oc-Mo) (Oc refers to the corner oxygens) and n(Mo-Oe-Mo) (Oe refers to the edge oxygens) respectively. The red-shift of the bands of (C19H42N)3(PMo12O40) compared with those of PMA suggests some interaction between the cluster anions and the cationic headgroups of the surfactant. The IR spectrum of the TEOS-treated sample exhibits five absorption bands in the region between 1100 and 500 cm-1. It is different from the IR spectra of the PMA and (C19H42N)3(PMo12O40). The peaks of heteropoly acid near 800 and 880 cm-1 are overlapped by the strong absorption peaks of silicate xerogel which are near 800 cm-1. Only the peak near 960 cm-1 can show the existence of the heteropoly acid. After the extraction of the template with HCl/EtOH, the IR spectrum corresponds well to the product before the extraction, showing the structure remains intact.

Fig.1 Infrared spectra of (a)
PMA, (b) (C19H42N)3(PMo12O40), (c)
the TEOS-treated sample without extraction , (d) the solvent-extracted sample
3.2 X-ray diffraction
The XRD
pattern of the (C19H42N)3(PMo12O40)
is shown in Fig.2, and the (hkl) indexes are also indicated with the corresponding
diffraction peaks. There are several sharp peaks at low angles (less than 10°), while the
XRD pattern only shows broad lumps at angles greater than 10°. These features are again reminiscent of MCM-41, which shows sharp low-angle lines and
diffuse high-angle lines, indicative of long-range
ordering of channels but a locally glassy aluminosilicate framework [2].
However, the peaks are different from those for MCM-41 but similar to those for the layer
compounds at low angles [2,3,9,10], which indicates that the salt has the layer
structure. The interlamellar distance can be calculated from the 001 index as d001=3.00nm.
On the basis of CPK modeling, a fully extended CTA ion has a length of 30Å; completely
coiled up it extends ca. 15Å. As the phosphomolybdate cluster size is ca. 10-11Å, a
space of ca. 14-19Å is left for the organic groups. This implies that the hydrocarbon
tails penetrate each other, as might be expected.
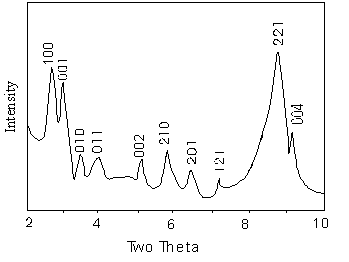
Fig.2 XRD pattern of (C19H42N)3(PMo12O40)

Fig.3 XRD patterns of (a) the TEOS-treated sample, (b) the solvent-extracted sample
The XRD patterns of the
TEOS-treated sample and the solvent-extracted sample are shown in Fig.3. The peak near
2.9° changes to 3.4° for the TEOS-treated sample, which means the interlamellar distance
decreases when treated with TEOS. And the strength of the peaks at low angles becomes less
sharp than the salt. It is contrast to the increase surface area of the material. We can
deduce that the addition of Si leads to the bending of the layers, and at last results in
the transformation of the lamellar salt into a microporous structured compound through the
interaction between the surfantant / heteropoly-acid and the silicate.
3.3 Atomic force micrograph
The Atomic Force Micrograph in Figure 4 showed the fine structure of the powder.
Clearly the solvent-extracted sample has the structure of uniform pores. The pore average
diameter, which was determined by the AFM observation, was 35 nm. The XRD and AFM
measurements reveal that the TEOS-treated sample after solvent extraction is a mesoporous
structured material.
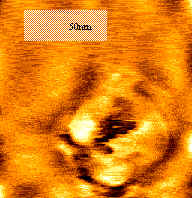
Fig.4 Atomic Force Micrograph of
the solvent-extracted sample
3.4 BET surface area
With the presence of the surfactant, the TEOS-treated
sample was nonporous (0.05 m2/g). After partial removal of the template, the
surface area increases to 140 m2/g. The bands around 3000 and 1500 cm-1
assigned to the stretching and bending vibrations of C-H of the surfactants are observed
in the IR spectrum, indicating that some surfactant was still present and the extraction
was uncompleted. Even in the elementary analysis the component of the element N decreased
from 1.02% to 0.77%. If HCl/EtOH extraction is complete, the BET surface area may be more
than 140 m2/g. A particle size determination by AFM shows an average diameter
of 2 mm, indicating that the high surface area cannot be attributed to the size of small,
dense spherical particles.
The "salt-gel" process was used to connect prestructured cluster arrays. The porous structures with high surface areas were obtained after the surfactant template was partially removed from TEOS-treated phosphomolybdate salts by solvent extraction. IR and XRD data indicate that silicate was indeed incorporated in the salt structures and the heteropoly structure remained intact.
The hydrolysis product of the tetraethyl orthosilicate is the SiO2 xerogel. When phosphomolybdic acid was hydrothermally reacted with TEOS at 368K, the quantity of the water (mostly molecular water and crystal water) is very small, the molar ratio of the water and the TEOS is less than 0.05:1, in which the PMA is the acid catalyst. The whole system is heterogeneous, while the hydrolysis is homogenous.In the system there is no deposit produced. The hydrogen bonds on the surface of SiO2 xerogel are few, hence the product SiO2 has no intense linkage with the PMA.
The surfactant salts of phosphomolybdic acid reacted with TEOS is a “salt-gel” reaction. In the reaction water is little and TEOS is hydrolyzed, finally the products of large BET surface area are obtained. It indicates that firstly PMA reacted with cationic surfactants get a salt with stratification structure, the hydrolysate SiO2 of TEOS can be absorbed into the interlaminar salt by the organophilic part (C19H42N+) of the surfactants, then linking with bridge oxygen to form Mo-O-Si bond and results inner pore structures in the phosphomolybdic acid. REFERENCES
[1] Behrens P. Adv. Mater, 1993, 5: 6.
[2] Kresge C T, Leonowicz M E, Routh W J et al. Nature, 1992, 359: 710.
[3] Beck J S, Vartuli J C, Roth W J et al. J. Am. Chem. Soc., 1992, 114: 10834.
[4] Akira T, Takayuki A, Masakazu I. Microporous and Mesoporous Materials, 1998, 21: 387.
[5] Yang P, Zhao D, Margolese D I et al. Nature, 1998, 396: 152.
[6] Stein A, Fendorf M, Jarvie T P et al. Chem. Matter, 1995, 7: 304.
[7] Janauer G G, Dobley A, Guo J et al. Chem. Mater, 1996, 8: 2096.
[8] Rocchiccioli-Deltcheff C, Thouvenot R, Franck R. Spectrochim. Acta , 1976, 32A: 587.
[9] Ravikovitch P I, Wei D, Chueh W T, Haller G L et al. J. Phys. Chem., 1997, B101: 3671.
[10] Kruk M, Jaronic M, Sayari A. Microprous Mater, 1997, 9: 173.